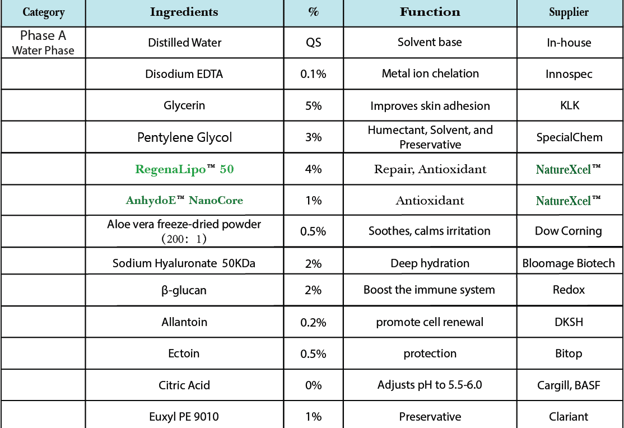
Phased Manufacturing Process
Phase I: Aqueous Phase Preparation (A-Phase)
Dissolving Difficult Components
Allantoin: Take 30% of formula water, heat to 70°C. Add allantoin and stir until clear. Cool to 40°C.
Beta-Glucan: Swell in warm water (40°C, 10% formula water) for 30 min to form gel.
Hydrolyzed HA: Disperse in 5x cold water and soak for 2 hours. Avoid high-speed stirring to prevent air entrapment.
Mixing Aqueous Phase
Sequence:
Birch Sap (70%) → Glycerin (5%) + Pentylene Glycol (3%) → Allantoin Solution → Beta-Glucan Gel
Add pre-dispersed HA → Ectoin (0.5%) → Pre-hydrated Aloe Powder (pre-mixed in cold water)
Maintain A-phase temperature at 35-40°C.
Phase II: Oil Phase & Active Ingredient Addition (B/C-Phase)
Component Handling Addition Condition
AnhydoE™ NanoCore No pre-treatment Add directly to A-phase (35°C)
RegenaLipo™ 50 Critical! Remove from fridge, equilibrate to 25°C Add last, low-speed stirring (≤200 rpm)
Note: RegenaLipo™ 50 contains 15% DMSO. High temperature or high-speed stirring will degrade actives. Must be added slowly at ≤30°C with stir speed ≤200 rpm.
Phase III: Homogenization & Finalization
Emulsion Homogenization
Transfer A-phase to homogenizer. Stir at 3000 rpm.
Slowly add B-phase (AnhydoE™).
Homogenize for 5 min, then cool to 25-28°C. Switch to paddle stirring (≤200 rpm).
Sensitive Active Addition
Slowly drip RegenaLipo™ 50 (maintained at 25°C) into mixture.
Stir for 10 min until uniform.
pH Adjustment & Preservation
Adjust pH to 5.0-5.3 (optimal for aloe activity) using 10% Citric Acid solution.
Add Preservative System (0.8%), stir for 15 min.
Key Process Control Points
Temperature Restrictions
RegenaLipo™ 50: Never expose to >30°C (prevents DMSO volatilization & liposome damage).
Aloe Powder: Dissolution water ≤40°C (prevents polysaccharide chain breakage).
Component Integrity
Beta-Glucan: Do not mix directly with high electrolytes (e.g., undiluted Ectoin) → Add after pre-swelling.
Citric Acid: Must be diluted to 10% to prevent localized acidity causing precipitation.
User Application Notes
Texture Optimization: For lighter feel, replace 1% Glycerin with Dipropylene Glycol.
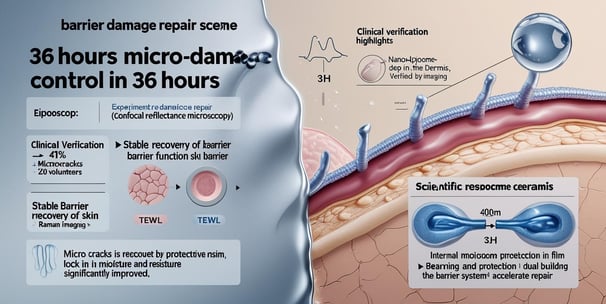
Efficacy Boost: Use with microcurrent device during clinical testing (enhances RegenaLipo™ penetration).
Risk Avoidance: Do not combine with ethanol or AHA-containing products (DMSO may increase irritation potential).
This process ensures >92% active ingredient retention while delivering a lightweight, non-greasy serum texture.
Test Parameter
pH Value
Viscosity
Microbiology
Active Retention Rate*
Standard
5.0-5.5
3,000-5,000 cP (25°C)
Complies with ISO 17516
≥90% (45°C/30 days)
Method
pH meter (calibrated at 25°C)
Rotational viscometer
Challenge test
HPLC (Glutathione in RegenaLipo™)
**Critical monitoring for Retinol & Glutathione stability in RegenaLipo™ 50.*